
(1)
Making non-emissive [6]cycloparaphenylene fluorescent by simple multiple methyl substitution
Tomoki Kato, Daiki Imoto, Akiko Yagi*, Kenichiro Itami*
Chem. Sci. 2025, Accepted. DOI: 10.1039/D5SC04694G
日本語の解説・加藤くんのコメント等はNewsへ!
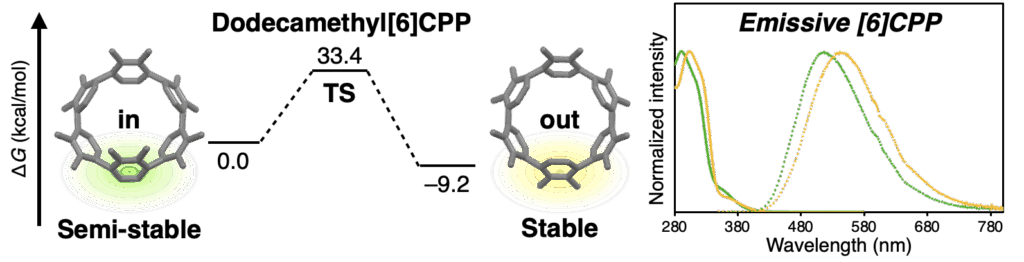
Abstract
We report the unexpected discovery that non-emissive [6]cycloparaphenylene ([6]CPP) can be made fluorescent by multiple additions of simple methyl groups. Dodecamethyl[6]CPP (Me12[6]CPP) was synthesized using a gold-based macrocyclization method and isolated as a pair of isomers (rotamers), both of which fluoresce at 510–540 nm. Experimental and theoretical investigations revealed that multiple methyl substitutions suppress rotation around the phenylene units, enabling the individual rotamers to be isolated and their interconversion to be studied. This simple yet significant “methyl effect” in CPPs not only enhances our basic understanding of stereoelectronic effects in CPPs, but should also facilitate the design and application of CPP-based materials in various fields.



